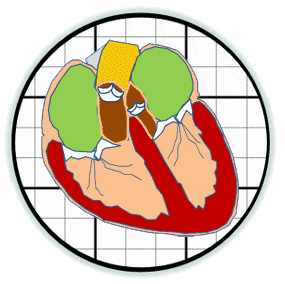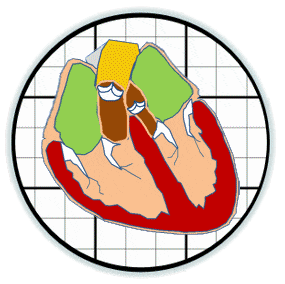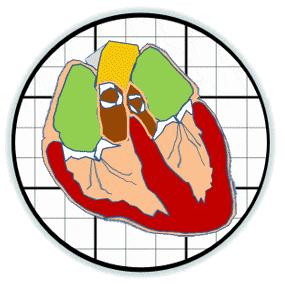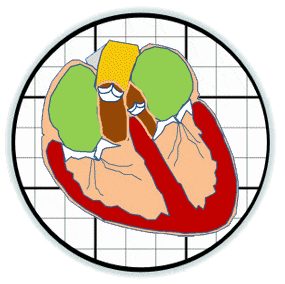
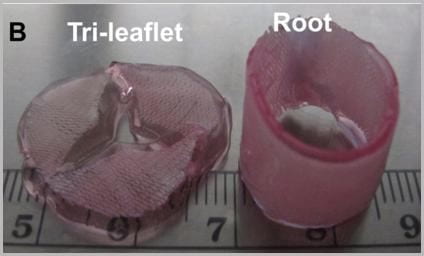
The prosthetic valves available to treat heart valve disease are poorly suited for younger and more active patients. Tissue engineering has tremendous potential for impact here by providing a living de novo graft capable of needed growth and integration. However, despite over 20 years of research, none are clinically ready. Recent efforts have focused on transcatheter delivery, which even if successful targets the very old and is not suitable for the young. We have pioneered 3D tissue printing technology to create anatomically accurate and mechanically heterogeneous fully cellularized living heart valve conduits. First, we have developed printable (extrudable), photocrosslinkable, biologically derived, cell-friendly hydrogel building blocks that attain mechanical properties of the root sinus and leaflets. Second, we have optimized the engineering control of soft tissue hydrogel printing, achieving a 200 μm minimum extrusion width and spatial precision of 15 μm. Third, we have developed and integrated an on-board photocrosslinking ultraviolet light-emitting diode (UV-LED) that rapidly cures tissues simultaneous with printing. Fourth, we have developed algorithms that process 3D image datasets of a native valve (e.g. CT or MRI) to automatically extract the valve geometry and segregate root and leaflet regions. These innovative technologies enable precise design of conduit macro-geometry to affect native-like valve tissue formation. We have further synthesized and characterized unique formulations of PEGDA, Alginate, gelatin, and hyaluronic acid, individually and blended, that are 3D printable. We have optimized formulations to mimic the physiological biomechanical range of human pediatric valves, and successfully fabricated cell-seeded 6-month-old sized valves. We have further demonstrated tunable stiffness (via altering molecular weights and blending), cell adhesion ligand density (via covalent conjugation of adhesion sequences like RGDS), and growth factor tethering. We recently showed that these components help maintain native root wall smooth muscle cell (SMC) and VIC phenotype in 3D culture. We also show that stiffness and adhesion ligand density control mesenchymal stem cells (MSC) differentiation towards these critical lineages for TEHV and away from calcification. We have also created a vector-based toolpath creation algorithm for achieving local variation in printed tissue composition with a small number of components. We believe these heterogeneous anatomically precise components are essential to improving the success of heart valve tissue engineering.
Selected Projects:
Hemodynamic stimulation of native and engineered heart valves
Heart valve structural composition and functional performance evolves over fetal and childhood development. How specific biomechanical forces promote proper valve growth, remodeling, and strengthening is poorly understood. Most of what is known is gathered from analysis of excised valves, but the performance of the living valve in different biomechanical environments is not well understood. We have developed a novel bioreactor technology capable of subjecting valved conduits to arbitrary ventricular and aortic pressure waveforms that mimic age and health specific conditions. We are studying how these parameters control valve tissue growth and remodeling in vitro, and whether engineered valves respond similarly to these conditions. These results will inform and validate hemodynamic stimulation regimens and functional targets for engineered valved conduits pre-implantation. This technology also enables accelerated pre-clinical evaluation of novel surgical approaches for living valve replacement before their evaluation in animal models.
Engineered Extracellular Matrix for Local Control of Valve Cell Differentiation
Heart valve tissue matrix is embedded with a constellation of smooth muscle and fibroblast like phenotypes whose coordination is essential for proper tissue function. The smooth muscle of the aortic root wall behaves very differently than the fibroblasts within the valve leaflets, which in turn is associated with local extracellular matrix environments that vary considerably in their structural composition and biomechanical properties. We are studying how features of the extracellular matrix milieu participate in regulating local cell phenotype. We use novel 3D tissue biofabrication techniques to replicate these heterogeneous matrix configurations and test how they direct differentiation of autologously accessible stem cells (e.g. bone marrow, fat tissue) towards valve phenotypes and promote interfacial integration. We use this information to guide the synthesis of novel biologically based polymers that are compatible with these biofabrication strategies for de novo valve conduit engineering.
Multi-modal Fabrication of Engineered Cardiovascular Tissues
The need for replacement cardiovascular tissues is great, in particular for pediatric patients suffering from complex congenital heart defects where major portions of their valves and great vessels need to be reconstructed. We have pioneered the use of 3D tissue printing to fabricate complex anatomical shapes. We continue to build on our bioprintable materials, deposition methods, and bioreactor technology to enable broader classes of material components to be deposited with higher spatial resolution without compromising clinically relevant sizing or cell viability. We are developing technology to replicate this structural heterogeneity and anisotropy directly during the printing and conditioning process. We are further developing quantitative analysis tools to define the mechanical properties of heart valves, which are essential for proper function in vivo. We are also applying novel innovations to print other soft tissues requiring complex macroscale geometry.
Biohybrid Engineering of Prosthetic Cardiovascular Replacements
Bioprosthetic tissue valves have enjoyed increased application in recent years because of their superior hemodynamic performance, but remain indicated only for the older patients because of their inevitable structural degeneration that is accelerated in younger, more active patients. Mechanical prosthetics rarely fail structurally, but instead suffer from clotting risks that necessitate the use of anticoagulation medication. Endothelial cells line all blood contacting surfaces and provide a natural regulation of coagulation. We have developed novel engineering strategies that enable endothelial cells to seed and remain adhered to the surfaces of prosthetic valves even within ultra-high fluid shear stresses. These monolayers express natural anticoagulant phenotypes under shear stress, and exhibit nearly 1000 fold less platelet activation compared to the current surface preparations. We are designing novel mechanical valve prosthetics that take advantage of this technology, and expanding their evaluation in ex vivo and in vivo contexts. We are also pursuing the adaptation of this technology to other cardiovascular applications.