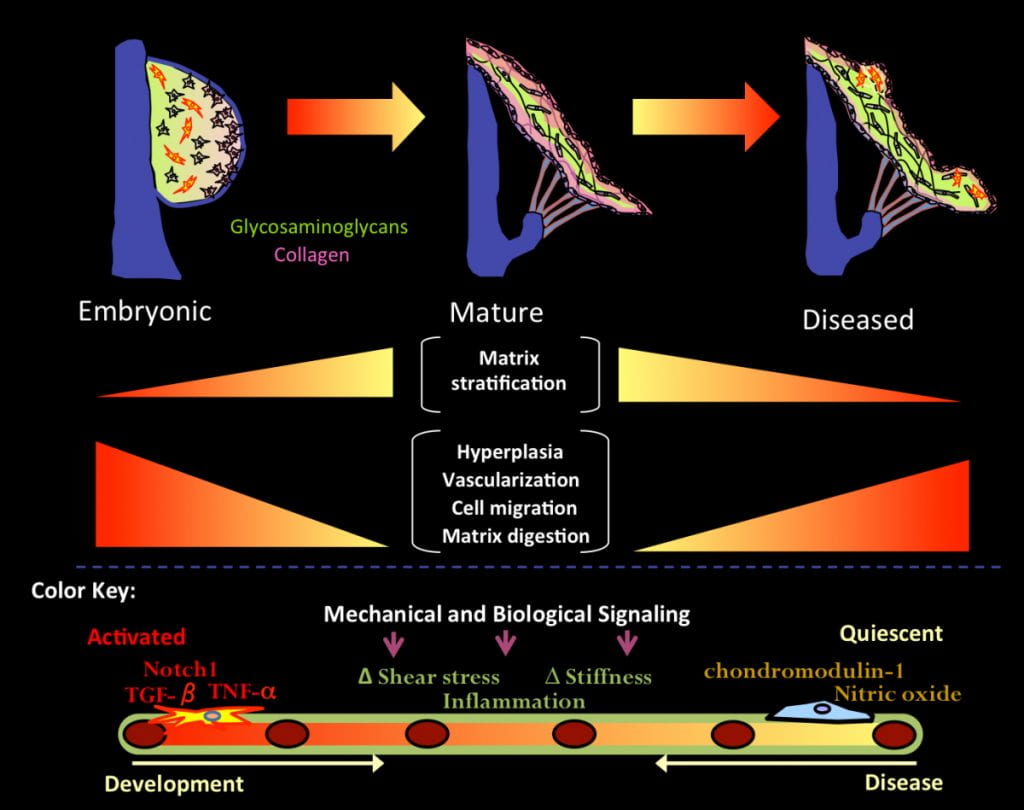
Heart valves are essential for maintenance of unidirectional blood flow and efficient cardiac output. Unlike atherosclerosis and coronary heart disease, heart valve disease is dramatically increasing in prevalence, severity, and medical expense, inflicts both young and old, is widely underdiagnosed, and burdens both the developed and majority world (the latter substantially). Valvuloseptal malformations are the most common and serious congenital heart defects (CHD), affecting 1-2% of all live births and contributing to 10-30% of preterm fetal demise. The exquisite function of heart valves over 2-3 billion cycles is only possible because of their unique heterogeneous biological and structural synergies. These features are naturally engineered during embryonic development through early postnatal life. How the early valve primordia (dubbed “cushions”) condense and elongate into thin fibrous valves and septa remains largely a mystery, yet when not done correctly are a serious clinical concern. We have developed and pursue a unique strategy that integrates developmental biology and innovative engineering expertise to test unique hypotheses that are complementary to the predominantly genetic approaches pursued in the field. We test how mechanical forces orchestrate heart valve morphogenesis, maturation, homeostasis and disease pathogenesis through the coordinated activities of resident valve endothelial and interstitial cells in their local 3D environment. We believe the critically missing mechanistic insight lies within these irreducible interactions, and therefore require new experimental, computational, and quantitative in vivo tools to elucidate. Furthermore, we believe that this natural engineering program for heart valves is critical to replicate for regenerative medicine strategies to advance.
Our research program has developed and applied innovative advances in theoretical, computational, cellular, tissue engineering, molecular, biomechanical, and imaging technologies to study heart valve development, physiology and pathology. We have further engineered novel device technology, including bioreactors, medical devices, and 3D tissue printing. We have dramatically expanded and shared the tools available for investigation in this complex field. We have also assembled a rich collaborative network of complementary experts that enhance our current and future impact potential. We are therefore well positioned to address these critically important but complex challenges. Our research program pursues three thrusts that iteratively inform and feedback to each other as described below. This fertile environment enables us to directly apply and translate the basic science and engineering we generate towards clinical benefit. Our trainees benefit directly from this intrinsically interdisciplinary and cross-collaborative environment to be well prepared for future careers in heart research, whether in academia, government, or industry.